
The first usable piles were studied in the Royal Society laboratories in London: W. Wollaston (1766-1828) perfected the “cup-pile” of Volta, using electrodes which may be extracted and an electrolyte with diluted sulphuric acid and nitrite.
In Paris the studies of many electrochemists lead to the invention of the lead accumulator (G. Planté) and of the pile with zinc-manganese bioxide (G. Leclanché). The Grenet pile, which used the couple zinc-carbon in a solution of bicromat potassium in sulphuric acid, was used in 1879 by Edison for domestic illumination.
Until recent times, the battery market was dominated by systems based on concepts from the last century. Also the rechargeable systems, that is electrochemical accumulators of common use, as nickel-cadmium and lead, operate on the basis of electrochemical processes proposed in the last part of the 19th century by W. Jungner and G. Planté.
Enormous progress was made recently with the utilization of lithium materials, for the productions of accumulators capable of energy densities at least three times larger than those of the lead accumulator.
1) Lead accumulator of the Plante’ type.
2) Fuel Cell, AEM, Milan – Bicocca.
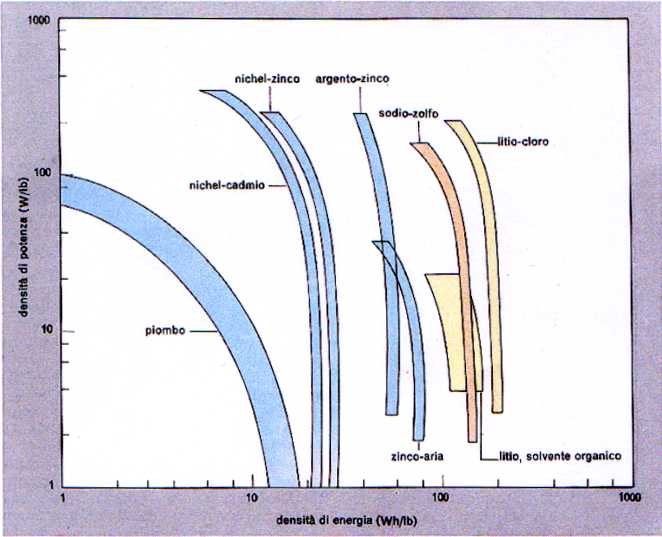
3) Operational features of several batteries.
Note that the highest energy densities are reached with lithium batteries (from Enciclopedia della Scienza e della Tecnica).

Battery (or pile), primary: The chemical reactions which take place during the discharge process are practically not-invertible: one may thus have only one discharge.
Battery (or pile), secondary: The chemical reactions which take place during the discharge process are invertible, and the element may be recharged when electric current flows in the direction opposite to that of the discharge.
Electric pile: Electrochemical device for the direct transformation of chemical energy into electrical energy.
Fuel cell: The difference between piles and batteries lies in the different location of the reactive substances: the fuel (hydrogen, hydrocarbons) and the oxidant (oxygen, air, chlorine) are located in appropriate tanks outside the cell.